A researcher with the University of Houston Cullen College of Engineering is a co-investigator on grants covering surviving a submarine escape and understanding the basic mechanisms of embryonic heart development.
While the two projects have little in common at first glance, they both rely on technologies and devices developed by Kirill Larin, associate professor of biomedical engineering and director of the college’s Biomedical Optics Laboratory.
The first project, on submarine escapes, is a collaboration with Marlowe Eldridge and Aleksey Sobakin, both from the University of Wisconsin. The team received a three-year, $1.1 million grant from the U.S. Navy’s NAVSEA program to develop protocols for avoiding decompression sickness during a submarine escape.
Decompression sickness, also known as the bends, occurs in people who go from an environment with high air pressure to one with lower pressure too quickly – the exact situation submariners experience during a rapid ascent. This change causes nitrogen that was dissolved in the blood to form gas bubbles.
“Decompression sickness can be very serious depending on where the bubbles form,” said Larin. “It can cause brain damage, paralysis and even death.”
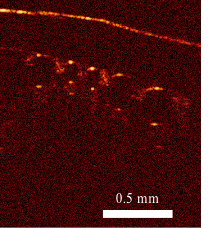
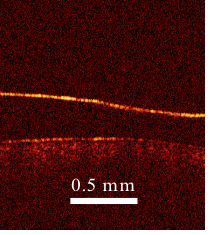
The investigators in Wisconsin will test different protocols for depressurizing using animal models. Larin’s contribution to the project is the development of a laser-based tool that can image the blood vessels that feed the brain, where bubbles can cause the most severe damage. With this tool, researchers will be able to detect whether and how many nitrogen bubbles form in these vessels under different depressurization conditions.
Based on their findings, the team will develop principles that will enable a safer rapid ascent, including when those rising to the surface should be given a breath of pure oxygen (known as a pre-breath) and when the ascent should briefly pause. While the grant only covers the development of these principles, future work could focus on programming these principles, in the form of algorithms, into the Navy’s submarine escape vessels.
Larin is also a collaborator on two grants aimed at imaging how the cardiovascular system develops in animal model embryos. One project, led by Irina Larina from Baylor College of Medicine (BCM), is supported by a five year, $1.95 million grant from the National Institutes of Health (NIH). In this effort, Larin’s optical imaging technology is being used to examine one key aspect of embryonic heart development: whether a developing heart actively sucks blood in prior to pumping it out or whether the blood is simply squeezed into the heart by motion of the heart wall.
The other project, led by BCM’s Monica Justice, is funded by a three-year, $25.55 million NIH grant. This effort is aimed at understanding fetal development and disease development on a very broad scale. Larin’s responsibilities include examining animal model cardiovascular systems after random genetic mutations.
Both rely on an optical imaging tool developed by Larin that shoots photons into the embryonic tissue. Based on the reflectivity of that tissue and how long it takes the photons to bounce back to their source, Larin is able to create an image of the developing cardiovascular systems.
Each project, said Larin, will provide researchers with a much deeper understanding of how the cardiovascular system is formed. This knowledge should help researchers and physicians understand when and why something goes wrong during cardiovascular development.
“This can help us understand congenital heart defects,” said Larin. “If we know more about how the heart forms and how it operates in terms of dynamics and structure and function, the more we’ll be able to predict and treat congenital diseases.”