Zeolites play an important role in the chemical and petroleum industries. These crystalline materials are used as catalysts that set in motion and/or improve chemical processes that create thousands of commercial and consumer products.
Despite their importance, the way zeolites themselves are usually synthesized is surprisingly ad hoc. When developing the chemical reactions that result in zeolite formation, researchers often fail to explore design parameters, such as the ideal temperature or appropriate compositions. Moreover, few researchers have made a serious effort to examine the concerted effects of multiple parameters to create a deeper understanding of zeolite synthesis.
Until now, that is. Jeff Rimer, assistant professor of chemical and biomolecular engineering with the University of Houston Cullen College of Engineering, has published a journal article that outlines a method for improving the synthesis of certain zeolites and proposes a new, graphical method of systematically tailoring zeolite crystallization.
The article, “Controlling Crystal Polymorphism in Organic-Free Synthesis of Na-Zeolites” appeared in the February 20 issue of the Journal of the American Chemical Society (JACS). This work was coauthored with Matthew Oleksiak, a graduate student in Rimer’s group; Miguel Maldonado, a UH undergraduate student; and Siva Chinta, a Ph.D. researcher at Total Petrochemicals. The article was featured on the journal’s cover and was chosen as a JACS Spotlights article.
The title of this study refers to Rimer’s efforts to improve the production of organic-free zeolites, which are cheaper to synthesize than those that use organic materials in the production process. They are, however, more likely to form polymorphs, which are crystals with identical composition but different structure at the molecular level. The presence of polymorphs, or impurities, can have a negative impact on the efficiency of zeolite catalysts compared to more uniform and pure crystals.
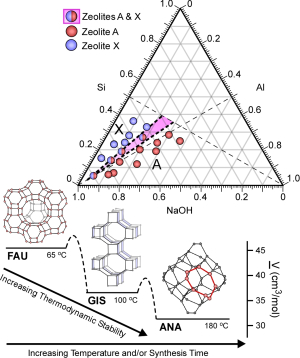
Just as important as these findings, though, was how Rimer presented them. While most zeolite research offers a simple formula that describes how the catalyst was prepared, Rimer employed a three-dimensional diagram that shows how multiple synthesis parameters, such as time, temperature, and the different concentrations of the chemicals used to create the zeolites, lead to the formation of pure crystal structures.
“When you look at these diagrams, what you are seeing are regions where pure crystal structures form, and regions where polymorphs are present,” said Rimer. “This allows us to graphically map out a parameter space to achieve different zeolite structures.”
On its own, this paper serves as an interesting way to present a single zeolite study. Rimer’s goal, however, is for investigators to widely adopt this diagram when presenting their zeolite work. If this occurs, Rimer said, researchers should be able to study multiple diagrams to determine an optimal set of parameters to create new zeolite structures and/or compositions.
That’s not the end of their usefulness, though. These ternary diagrams can also help researchers understand the mechanism of zeolite formation. Given the ad-hoc nature of most zeolite research, Rimer said, the actual processes by which zeolites nucleate and crystallize are largely unanswered. This more rigorous approach to zeolite research can help uncover these answers.
“The first step is to establish a more systematic, scientific approach to studying this process,” he said. “The next level is to identify patterns in crystal formation and then figure out how to explain and predict these trends. That’s the more challenging aspect, but I also think it’s the most interesting.”